Abstract
Introduction - Data regarding efficacy and toxicity of different bridging strategies prior to CAR-T therapy are scanty. Tisagenlecleucel (Kymriah TM, Novartis) and axictagene ciloleucel (Yescarta TM, Kite/Gilead) were commercially approved for relapsed/refractory (R/R) DLBCL since 2019. We analyzed real-life data of CAR-T therapy among all consecutive patients who were treated in 4 different CAR-T centers in Israel.
Methods - From May 2019, 144 R/R DLBCL patients underwent apheresis and continued to receive bridging therapy that included chemo/immunotherapy (n=78, 54%), radiation (n=11, 7.6%), chemoradiation (n=22, 15%), steroids only (n=5, 3.5%) and none (n=28, 19.4%). All patients were evaluated after bridging therapy and prior to CAR-T infusion by PETCT (96%) or CT scan (4%).
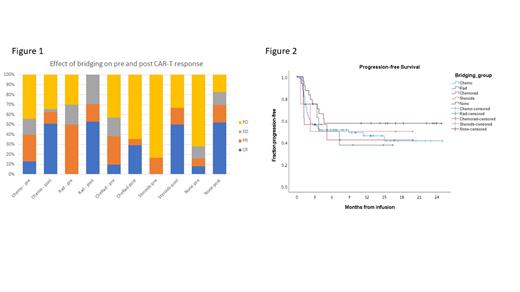
Results - Median age was 68 (20-88) years and Median follow-up was 13 (4-26) months. All 144 patients underwent successful apheresis. Reasoning for choosing specific bridging therapy was based on low tumor mass (n= 23, 16%), high tumor mass (n=73, 51%), frailty of the patient (n=27, 19%), ongoing significant prior regimen's toxicities (n=14, 10%) and local disease (n=7, 4%). In patients given radiation therapy median dose was 23 (range, 8-30) Gy. In patients given chemo/immunotherapy or chemoradiation, sepsis was the main complications (9% of all patients) during bridging therapy. However, none of the patients had a fatal event. 14 patients (9.7%) did not proceed to CAR-T infusion; 6 (4.2%) had disease progression and died; 8 (5.6%) had manufacture failure). Among the 130 patients that received CAR-T infusion, PET-CT prior to preparative regimen demonstrated CR/PR status in 38%, 50%, 40%, 17%, and 16% of patients given chemotherapy, radiation, chemoradiation, steroids only, or no bridging therapy, respectively (p=.15), Figure 1. Any bridging therapy was associated with a better disease control compared to either steroids only or no treatment (p=.012). There were no differences in the incidences of overall CRS (p=.692), grade 3-4 CRS (p=.196), overall ICANS (p=.941), grade 3-4 ICANS (p=.281), acute kidney disease (p=.244), and liver dysfunction (p=.45) between the 5 different bridging strategies. Cardiovascular complications were more common after chemoradiation (36%), chemotherapy (19%) and radiation (13%), compared with steroids (0%) or no bridging therapy (4%), p=.05. Non-relapse mortality was 0 in all subgroups. PETCT at 1-month post CAR-T infusion demonstrated an increase in CR status percentage across all subgroups with no statistically significant difference in the incidence between the subgroups (p=.27), Figure 1. There was no difference in both progression-free survival (Figure 2) and overall survival between the 5 subgroups (p=.7, and p=.23). Cox regression model identified preinfusion lower ECOG status (HR=0.8, p=.04), preinfusion CR/PR status (HR=.46, p=.037) and 1-month post infusion CR status as a time dependent co-variate (HR=.14, p<.01) to be associated with better progression-free survival, while age and type of bridging therapy did not predict survival.
Conclusions - Bridging to CAR-T should be tailored based on patient's and disease's characteristics with the aim to achieve the best disease control prior to CAR-T. However the chosen strategy per-se does not impact long-term outcomes. Intensive bridging therapy is associated with more cardiovascular events after CAR-T infusion. A prospective-controlled-trial allocating patients to different bridging strategies is needed to verify these results.
Ram: Gilead: Honoraria; Novartis: Honoraria. Yehudai-Ofir: Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees. Avivi: Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau. Zuckerman: Cellect Biotechnology: Honoraria; Gilead Sciences: Honoraria, Speakers Bureau; Novartis: Honoraria; Janssen: Honoraria; BioSight Ltd: Honoraria; Orgenesis Inc.: Honoraria; AbbVie: Honoraria. Yeshurun: Astellas: Consultancy; Janssen: Consultancy. Gurion: Medison; Gilead Sciences; Takeda Pharmaceuticals: Consultancy; JC Health CARE; Roche: Honoraria. Levi: AbbVie: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal